菜单
CN | CNY
CN | CNY

It’s Not Just About Size: Talking about Shake Flasks and Bioreactors
实验室学院
- 生物处理
- 细胞培养
- 质量
- 可扩展性
- 自动化
- 生物过程工艺
- 文章
Shake flasks and bioreactors are both important tools for bioprocessing, but are very different in many respects. Bioreactors clearly offer a much wider range of capacities, but more interesting are the differences in overall performance, the distinctive functionalities offered, and the consequent benefits for particular applications.
Easy, fast, and inexpensive
Shake flasks have been used for decades to cultivate bacteria and fungi, as well as plant and animal cells in suspension. Protocols have been established for all common organisms, even the more unusual ones. It is hard to imagine a biotechnology lab in industry or research that doesn't use shake flask cultures. They are an easy-to-use and inexpensive choice for basic applications, such as organism screening, media design, and early process development. All that is needed is a flask and a shaker to move it. The user doesn't need technical knowledge, advanced equipment or sensing and control technologies.This very simplicity, however, is the biggest disadvantage. Shake flasks do not provide detailed insight into culture performance or the opportunity to monitor and control the process parameters.
Shaken not stirred
显示更少
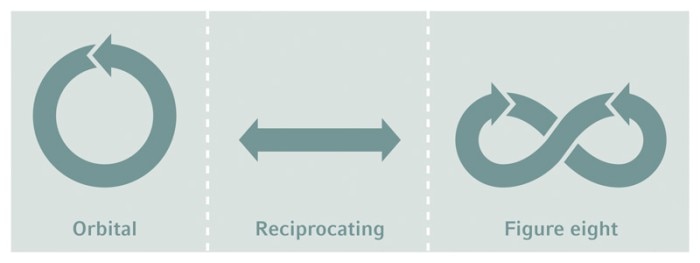
Orbit types
James Bond® knew it. There are differences between shaking and stirring, and not just for Martinis® . While bioreactor cultures are stirred for mixing, shake flasks are agitated on shakers, usually with an orbital motion, but sometimes in a reciprocating or figure-eight mode.
The agitation mode, direction, and speed influence the fluid dynamics, and therefore the heat transfer and mass transfer in the culture, resulting in a specific performance.
The agitation mode, direction, and speed influence the fluid dynamics, and therefore the heat transfer and mass transfer in the culture, resulting in a specific performance.
显示更少
Fluid dynamics
Beside temperature and nutrient supply, oxygen availability is vital to microbial and cell culture growth. In shake flasks, the oxygen transfer takes place via two liquid surfaces: the bulk liquid surface and the liquid film on the wetted shake flask wall. The oxygen transfer rate (OTR) in shake flasks is determined by the flask size and shape, agitation speed, filling volume, and ambient conditions.
Limited insight and control
Cultivation processes in shake flasks are never under true control. These systems do not allow for online measurements of critical process parameters such as temperature, dissolved oxygen or optical density, because the flasks cannot be easily equipped with sets of sensors. Direct control of these parameters is also impossible. Temperature and oxygen, for example, can only be regulated by controlling ambient conditions in a closed shaker system or a cultivation room. Automated processes, such as culture feeding according to defined profiles or the integration of control loops, cannot be realized. Controlling a bioprocess in shake flasks would involve a lot of manual work in taking samples, making offline measurements, and adjusting levels manually.Shaking has its place
显示更少
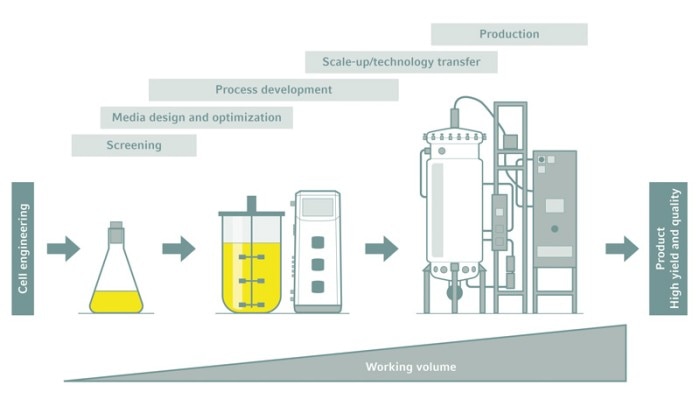
From shake flask to production
Shake flasks are suitable for screening, media optimization, and early process development. Whenever it comes to implementing a robust bioprocess or switching to a larger scale production, however, they cannot keep up.
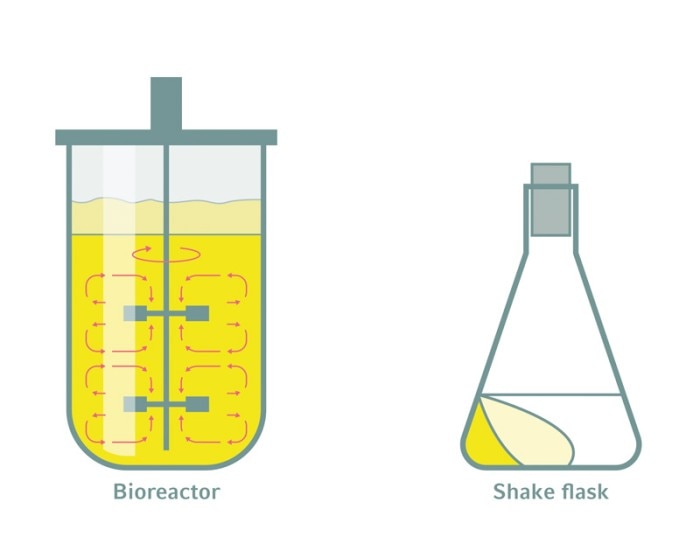
Bioreactors are closed systems equipped with multiple sensors, motor-driven impellers for agitation, and instruments for temperature control, gassing and the supply of liquids. Associated software facilitates online monitoring and control of all relevant process parameters, and often allows for integrated analytics and comprehensive data processing as well.
Small volumes make little product
It’s obvious that the amount of product made depends on the product titer and the working volume. But the solution to making more product is not simply to increase the process volume. Scaling up a bioprocess requires the use of bioreactors that allow for predictable scalability.Bioreactors are closed systems equipped with multiple sensors, motor-driven impellers for agitation, and instruments for temperature control, gassing and the supply of liquids. Associated software facilitates online monitoring and control of all relevant process parameters, and often allows for integrated analytics and comprehensive data processing as well.
Real-time monitoring enhances process understanding
显示更少
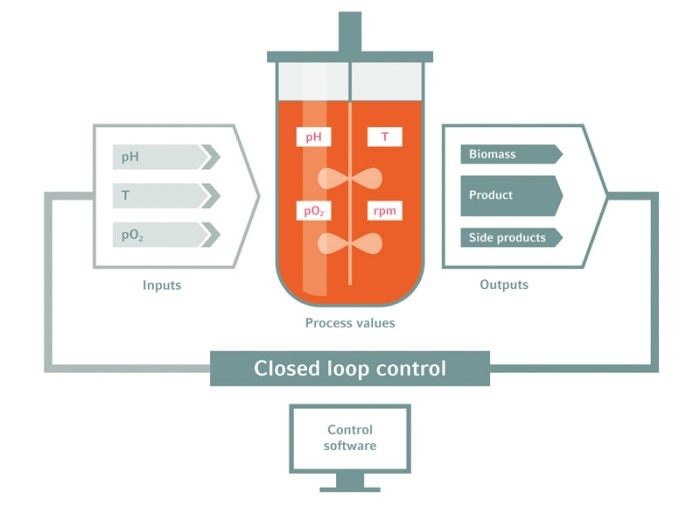
Bioreactor systems offer real-time process control
Bioreactors are equipped with multiple online sensors that allow for real-time observation. Temperature, dissolved oxygen or biomass are measured constantly and displayed numerically and graphically. Exhaust, metabolites, and redox potential can be calculated from the measured process parameters. Process protocols, set-points, feeding and gassing profiles, and more can be pre-programmed. The bioreactor control units then facilitate the interaction of hardware and software to enable precisely controlled bioprocessing according to predefined protocols.
This combination of real-time monitoring, precise control, and detailed reporting changes the black-box of a shake flask cultivation into in-depth bioprocess insight.
This combination of real-time monitoring, precise control, and detailed reporting changes the black-box of a shake flask cultivation into in-depth bioprocess insight.
All under control
显示更少
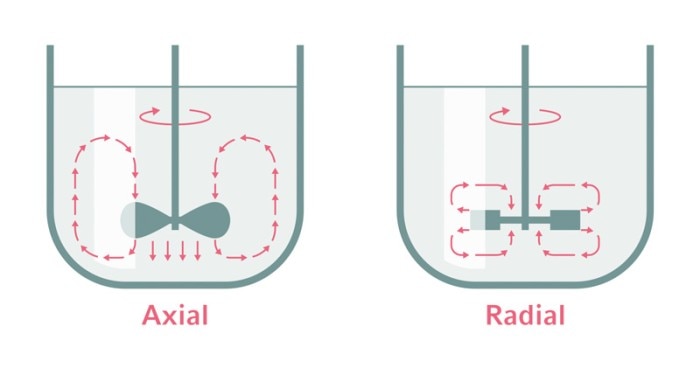
Different impeller types result in axial or radial mixing profiles.
Bioreactors can be used to cultivate microorganisms, plant, insect, and mammalian cells, and even very sensitive stem cells. The adaptability of bioreactor systems allows a broad range of applications. Various types of impellers operated at specific agitation speeds can address the individual requirements of different cell types regarding shear stress and mixing efficiency.
The availability of oxygen in a bioreactor is key to successful bioprocessing, as previously described for shake flasks. The OTR is influenced by the bioreactor design and other controllable parameters, including the bioreactor dimensions, impeller design, sparger type, agitation speed, gas flow rate, and gas concentration.
The maximum OTR in a bioreactor can be calculated by following formula:
The availability of oxygen in a bioreactor is key to successful bioprocessing, as previously described for shake flasks. The OTR is influenced by the bioreactor design and other controllable parameters, including the bioreactor dimensions, impeller design, sparger type, agitation speed, gas flow rate, and gas concentration.
The maximum OTR in a bioreactor can be calculated by following formula:
显示更少

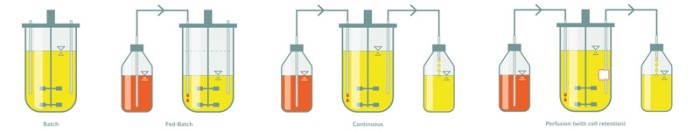
Culture modes in bioprocessing
Further technical options that support running different cultivation modes include the ability to feed the bioreactor culture, to change medium while the cells are retained, to measure the biomass online or simply to pump in and out the bioreactor. While batch and fed-batch cultivation are commonly used to optimize growth in microbial applications, continuous culture and perfusion modes work out well in animal and human cell culture, for example.
Fully functional, stirred-tank bioreactors are even available for working volumes of less than 100 mL, perfect for early bioprocess development. Bioreactors of increasing volume provide reliable scalability if the vessels have similar geometries, heat and mass transfer capabilities, monitoring and control possibilities, and documentation tools. This streamlines the process development laboratory work, and eases the production of regulatory conformance documentation throughout the whole process.
Bioreactors provide users with almost unlimited flexibility, functionality, and freedom of action, but cost much more and need well-educated operators. Shake flasks, however, also have a place for simple screening and media development.
Literature:
Büchs J: Introduction to advantages and problems of shaken cultures. Biochemical Engineering Journal 7:2 (2001) 91-98
Büchs J and Klockner W: Advances in shaking technologies.
Trends in Biotechnology 30:6 (2012) 307-314
Meier K, Regestein L, Büchs J: Correlation for the maximum oxygen transfer capacity in shake flasks for a wide range of operating conditions and for different culture media. Biochemical Engineering Journal 109 (2016) 228-235
References:
[1] DECHEMA Arbeitskreis Single-Use-Technologie: Empfehlung für Leachable-Studien Standardisierter Zellkulturtest zur Identifizierung kritischer Filme. Januar 2014
[2] U.S. Pharmacopeial Convention: In-Process Revision: <661.3> Plastic Components and Systems Used in Pharmaceutical Manufacturing. In: PF 42(3), May-June 2016
[3] Denise Bestwick and Raymond Colton: Extractables and Leachables from Single-Use Disposables. Supplement to BioProcess International, February 2009
[4] Angelo DePalma: Extractables and Leachables: Standardizing Approaches to Manage the Risk. BioProcess eBook Series 2017, 15(3)E, March 2017
[5] Kevin A. Lannon et al.: Quantitative Risk Assessment of Bioaccumulation Attributes to Extractables and Leachables in Cellular Immunotherapy Biomanufacturing. BioProcess International, 13(10), November 2015
Going great—scaling up with bioreactors
Industrial-scale bioprocesses require the use of bioreactors. The challenge is to scale up the production process from small-scale development to bench scale, and then to pilot and production scales. Being able to optimize the process for product formation, quality, and yield at a small scale and then scale it up with minimal adjustments to production scale can save a great deal of time and money.Fully functional, stirred-tank bioreactors are even available for working volumes of less than 100 mL, perfect for early bioprocess development. Bioreactors of increasing volume provide reliable scalability if the vessels have similar geometries, heat and mass transfer capabilities, monitoring and control possibilities, and documentation tools. This streamlines the process development laboratory work, and eases the production of regulatory conformance documentation throughout the whole process.
Comprehensive software
The advantages of bioreactor processes over shake flask cultivations for complex bioprocesses are all the more convincing when bioprocess management software is applied. Special software solutions can interconnect multiple laboratory devices and help to automate the process. They can apply Design of Experiment (DoE) and process modeling, can support comprehensive data analysis, and can connect to historians and central data storage tools. Process Analytical Technology (PAT) approaches and Quality by Design (QbD) are facilitated.Shake flasks vs bioreactors?
No, there is no competition. It’s a question of objectives—each system has its own applications and advantages.Bioreactors provide users with almost unlimited flexibility, functionality, and freedom of action, but cost much more and need well-educated operators. Shake flasks, however, also have a place for simple screening and media development.
Literature:
Büchs J: Introduction to advantages and problems of shaken cultures. Biochemical Engineering Journal 7:2 (2001) 91-98
Büchs J and Klockner W: Advances in shaking technologies.
Trends in Biotechnology 30:6 (2012) 307-314
Meier K, Regestein L, Büchs J: Correlation for the maximum oxygen transfer capacity in shake flasks for a wide range of operating conditions and for different culture media. Biochemical Engineering Journal 109 (2016) 228-235
References:
[1] DECHEMA Arbeitskreis Single-Use-Technologie: Empfehlung für Leachable-Studien Standardisierter Zellkulturtest zur Identifizierung kritischer Filme. Januar 2014
[2] U.S. Pharmacopeial Convention: In-Process Revision: <661.3> Plastic Components and Systems Used in Pharmaceutical Manufacturing. In: PF 42(3), May-June 2016
[3] Denise Bestwick and Raymond Colton: Extractables and Leachables from Single-Use Disposables. Supplement to BioProcess International, February 2009
[4] Angelo DePalma: Extractables and Leachables: Standardizing Approaches to Manage the Risk. BioProcess eBook Series 2017, 15(3)E, March 2017
[5] Kevin A. Lannon et al.: Quantitative Risk Assessment of Bioaccumulation Attributes to Extractables and Leachables in Cellular Immunotherapy Biomanufacturing. BioProcess International, 13(10), November 2015
显示更少
